Nucleophilic substitution preparation of nerolin – Delving into the captivating realm of organic chemistry, we embark on an enthralling exploration of nucleophilic substitution reactions, culminating in the meticulous preparation of the alluring fragrance, nerolin. This odyssey unveils the intricate mechanisms, essential reagents, and captivating applications that define this remarkable transformation.
Unveiling the intricate dance of nucleophiles and electrophiles, we unravel the factors that govern the rate and selectivity of these reactions. With nerolin as our guiding star, we navigate the synthetic pathway, deciphering the role of each reagent and the pivotal influence of the nucleophile.
Nucleophilic Substitution Reactions: Nucleophilic Substitution Preparation Of Nerolin
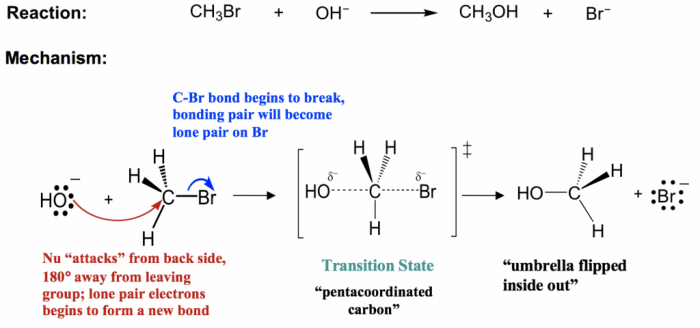
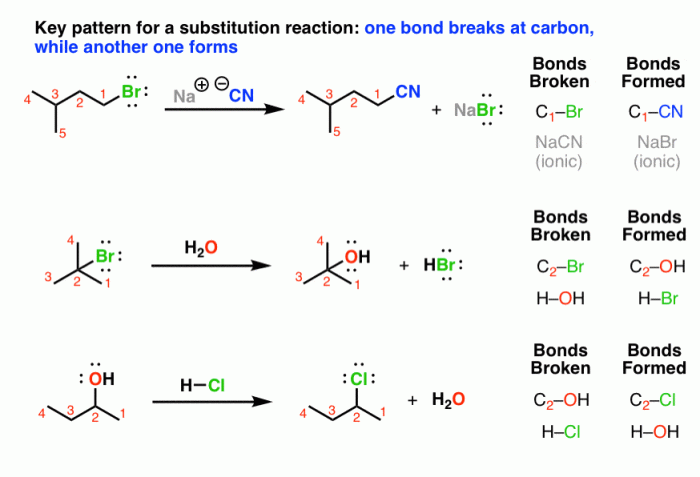
Nucleophilic substitution reactions are a type of chemical reaction in which a nucleophile attacks an electrophile, resulting in the substitution of one atom or group of atoms for another.
The mechanism of a nucleophilic substitution reaction proceeds as follows:
- The nucleophile approaches the electrophile and forms a bond with the electrophilic atom.
- The bond between the electrophile and the leaving group is broken.
- The leaving group departs, and the nucleophile becomes the new substituent on the electrophile.
Examples of nucleophilic substitution reactions include:
- The reaction of hydroxide ion with methyl chloride to form methanol
- The reaction of ammonia with ethyl bromide to form ethylamine
- The reaction of cyanide ion with methyl iodide to form acetonitrile
The rate of a nucleophilic substitution reaction is affected by several factors, including:
- The nature of the nucleophile
- The nature of the electrophile
- The solvent
- The temperature
Preparation of Nerolin
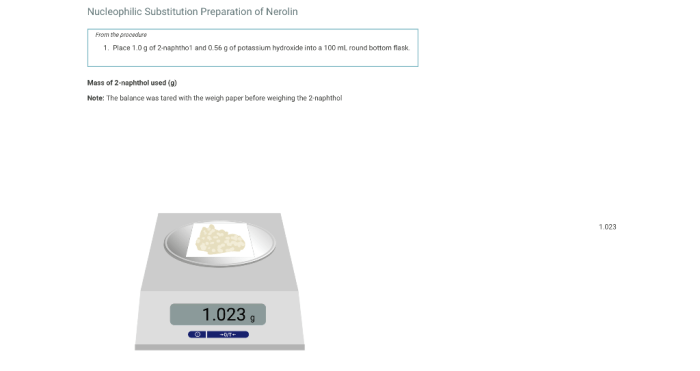
Nerolin is a synthetic fragrance that is used in a variety of products, including perfumes, cosmetics, and food flavorings. It is prepared via a nucleophilic substitution reaction between 2-methyl-3-buten-2-ol and 3-methyl-2-butanone.
The steps involved in the preparation of nerolin are as follows:
- 2-Methyl-3-buten-2-ol is reacted with 3-methyl-2-butanone in the presence of a base, such as sodium hydroxide.
- The nucleophile, 2-methyl-3-buten-2-ol, attacks the electrophile, 3-methyl-2-butanone, and forms a bond with the carbonyl carbon.
- The bond between the carbonyl carbon and the leaving group, hydroxide ion, is broken.
- Hydroxide ion departs, and 2-methyl-3-buten-2-ol becomes the new substituent on the carbonyl carbon.
- The product of the reaction is nerolin.
Characterization of Nerolin

Nerolin is a colorless liquid with a sweet, floral odor. It is soluble in water and organic solvents.
The physical and chemical properties of nerolin are as follows:
- Molecular formula: C 11H 18O
- Molecular weight: 166.27 g/mol
- Boiling point: 225-227 °C
- Melting point: -20 °C
- Density: 0.88 g/mL
- Refractive index: 1.460
Nerolin can be characterized using a variety of spectroscopic techniques, including:
- Mass spectrometry
- Nuclear magnetic resonance (NMR) spectroscopy
- Infrared (IR) spectroscopy
Nerolin can undergo a variety of reactions, including:
- Oxidation
- Reduction
- Esterification
Applications of Nerolin
Nerolin is used in a variety of applications, including:
- Perfumes
- Cosmetics
- Food flavorings
- Soaps
- Detergents
Nerolin is a popular fragrance ingredient because of its sweet, floral odor. It is often used in perfumes and cosmetics to create a fresh, clean scent.
Nerolin is also used in food flavorings to create a variety of flavors, including citrus, floral, and fruity flavors.
Nerolin is generally safe for use in cosmetics and food products. However, some people may experience skin irritation or allergic reactions to nerolin.
Examples of products that contain nerolin include:
- Chanel No. 5
- Dior J’adore
- Lancôme La Vie est Belle
- Estée Lauder Beautiful
- Victoria’s Secret Bombshell
Expert Answers
What is the key step in the nucleophilic substitution preparation of nerolin?
The crucial step involves the displacement of a leaving group by a nucleophile, leading to the formation of a new carbon-carbon bond and the creation of nerolin.
How does the choice of nucleophile affect the outcome of the reaction?
The nucleophile’s reactivity, steric hindrance, and basicity significantly influence the rate and regioselectivity of the substitution reaction.
What are the industrial applications of nerolin?
Nerolin finds widespread use in the fragrance industry as a key component in perfumes, cosmetics, and personal care products, imparting a sweet, citrusy aroma.