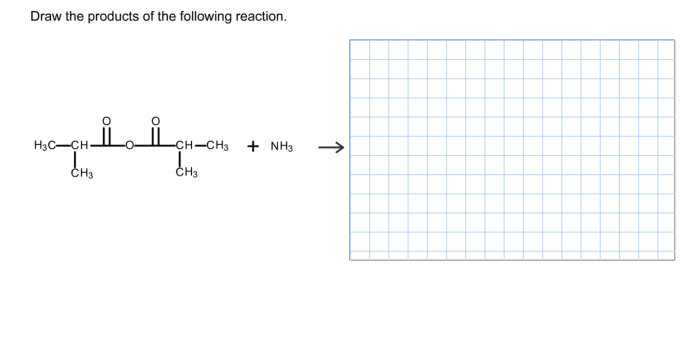
The periodic table section 3 reinforcement – The Periodic Table: Section 3 Reinforcement delves into the fascinating world of the periodic table, specifically focusing on Section 3 elements. This section of the table holds a treasure trove of elements that play pivotal roles in various chemical reactions and technological advancements.
Join us as we explore the properties, applications, and historical significance of these remarkable elements.
1. Introduction
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configuration, and recurring chemical properties. Section 3 of the periodic table consists of the d-block elements, which are characterized by having their d orbitals partially filled with electrons.
This reinforcement exercise aims to provide a deeper understanding of the elements in Section 3, their properties, chemical reactions, applications, and the trends and patterns observed among them.
2. Elements in Section 3: The Periodic Table Section 3 Reinforcement
Section 3 of the periodic table includes the following elements:
- Scandium (Sc)
- Titanium (Ti)
- Vanadium (V)
- Chromium (Cr)
- Manganese (Mn)
- Iron (Fe)
- Cobalt (Co)
- Nickel (Ni)
- Copper (Cu)
- Zinc (Zn)
These elements share similar chemical properties due to their similar electron configurations, which result in the presence of partially filled d orbitals.
Chemical Reactions Involving Section 3 Elements, The periodic table section 3 reinforcement
Section 3 elements participate in various chemical reactions, including:
- Oxidation-reduction reactions:These elements can undergo oxidation or reduction reactions, depending on their oxidation state and the reaction conditions.
- Complex formation:Section 3 elements can form stable complexes with ligands, which are molecules or ions that donate electrons to the metal ion.
- Catalytic reactions:Many Section 3 elements act as catalysts in various chemical reactions, facilitating the reaction process without being consumed.
4. Applications of Section 3 Elements
Section 3 elements have numerous practical applications due to their unique properties:
- Alloys:Section 3 elements are often used in alloys, which are mixtures of metals, to enhance their strength, hardness, and other properties.
- Magnets:Iron, cobalt, and nickel are essential components of magnets, which are used in various applications, such as motors, generators, and magnetic resonance imaging (MRI).
- Pigments:Chromium and copper compounds are used as pigments in paints, dyes, and ceramics.
- Batteries:Nickel and zinc are used in batteries, providing power for electronic devices and electric vehicles.
5. Trends and Patterns in Section 3
Several trends and patterns are observed in Section 3 of the periodic table:
- Atomic radius:The atomic radius generally decreases across Section 3 from left to right, due to the increasing effective nuclear charge.
- Ionization energy:The ionization energy generally increases across Section 3 from left to right, as it becomes more difficult to remove an electron from the partially filled d orbitals.
- Electronegativity:The electronegativity generally increases across Section 3 from left to right, indicating an increase in the ability of the elements to attract electrons.
6. Historical Significance of Section 3
The discovery and understanding of Section 3 elements have played a crucial role in the development of chemistry:
- Transition elements:The concept of transition elements, which include Section 3 elements, was first proposed by Dmitri Mendeleev in the 19th century.
- Atomic theory:The study of Section 3 elements helped refine the atomic theory and provided insights into the electronic structure of atoms.
- Quantum mechanics:The development of quantum mechanics provided a theoretical framework for understanding the properties and behavior of Section 3 elements.
Q&A
What is the purpose of Section 3 reinforcement in the periodic table?
Section 3 reinforcement provides a deeper understanding of the elements in Section 3, their properties, chemical reactions, and practical applications.
Which industries heavily rely on Section 3 elements?
Section 3 elements find widespread use in industries such as electronics, aerospace, medicine, and energy production.
How have Section 3 elements contributed to the advancement of science?
The study of Section 3 elements has played a crucial role in developing our understanding of atomic structure, chemical bonding, and reaction mechanisms.