Select the properties of the sn2 reaction mechanism – The study of the SN2 reaction mechanism delves into the intricate interplay of nucleophiles, electrophiles, solvents, and other factors that govern the rate and efficiency of this fundamental chemical process. Understanding the properties that influence the SN2 reaction mechanism empowers chemists to design and optimize reactions for a wide range of applications.
This comprehensive exploration of the SN2 reaction mechanism will elucidate the characteristics of nucleophiles and electrophiles, the impact of solvent polarity, steric effects, temperature, and leaving group ability. By unraveling these properties, we gain invaluable insights into the factors that shape the reactivity and selectivity of SN2 reactions, opening up new avenues for chemical synthesis and molecular design.
SN2 Reaction Mechanism: Select The Properties Of The Sn2 Reaction Mechanism

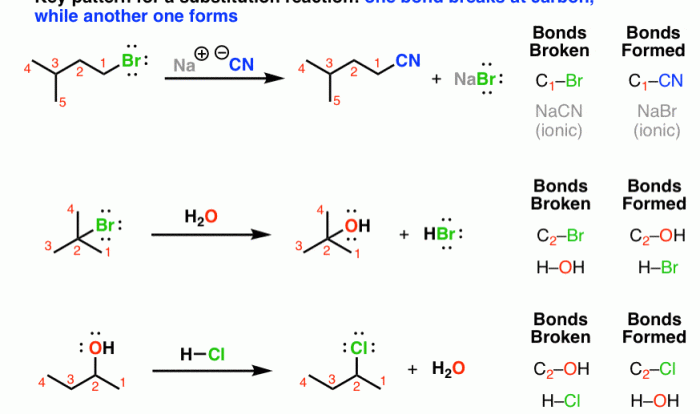
The SN2 (nucleophilic substitution, bimolecular) reaction mechanism is a fundamental organic reaction in which a nucleophile attacks an electrophile, leading to the substitution of a leaving group. This mechanism is characterized by its second-order kinetics, inversion of configuration at the reaction center, and the formation of a transition state with a pentavalent intermediate.
Nucleophile Characteristics
The strength of a nucleophile is a crucial factor in SN2 reactions. Strong nucleophiles, such as alkoxide ions or hydroxide ions, have a high electron density and are more likely to attack electrophiles. Conversely, weak nucleophiles, such as water or alcohols, have a lower electron density and are less reactive.
The size and charge of a nucleophile also affect its reactivity. Smaller nucleophiles are less hindered and can approach electrophiles more easily. Charged nucleophiles, such as anions, are generally more reactive than neutral nucleophiles.
Electrophile Characteristics
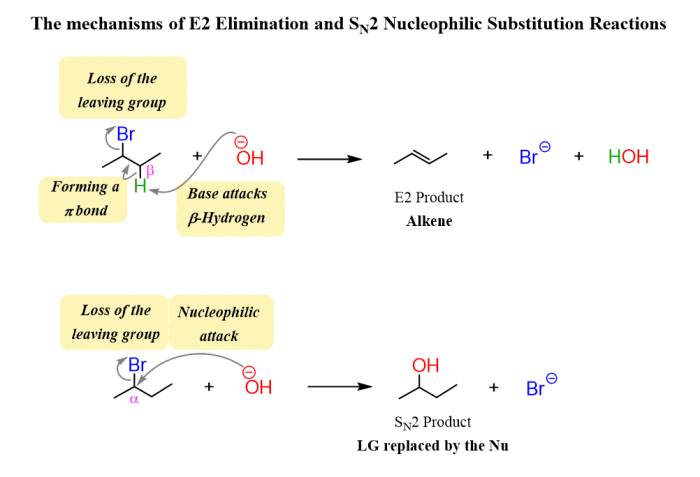
In SN2 reactions, the electrophile is the species that accepts the nucleophile. Ideal electrophiles are primary (1°) or secondary (2°) alkyl halides or alkyl tosylates, which have a good leaving group and a relatively unhindered reaction center.
Electrophiles with electron-withdrawing groups, such as carbonyl groups or cyano groups, are more reactive because they stabilize the developing positive charge in the transition state.
Solvent Effects

The polarity of the solvent plays a significant role in SN2 reactions. Polar solvents, such as water or dimethylformamide (DMF), stabilize the charged transition state and promote SN2 reactions. Conversely, nonpolar solvents, such as hexane or benzene, hinder SN2 reactions by not solvating the ions effectively.
Protic solvents, which have a hydrogen atom bonded to an electronegative atom, can also participate in hydrogen bonding with the nucleophile, reducing its reactivity.
Steric Effects
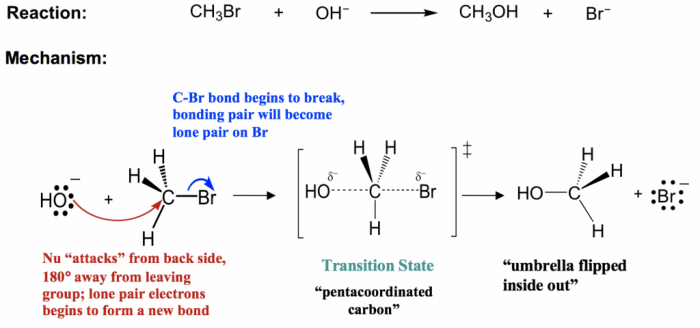
Steric hindrance refers to the presence of bulky substituents around the reaction center. Bulky substituents can hinder the approach of the nucleophile, reducing the reaction rate. The effect of steric hindrance is more pronounced in SN2 reactions than in other reaction mechanisms.
Temperature Effects
The rate of SN2 reactions increases with increasing temperature. This is because the higher temperature provides more energy to overcome the activation energy required for the reaction. The relationship between temperature and reaction rate is described by the Arrhenius equation.
Leaving Group Ability
The leaving group is the group that is displaced by the nucleophile in an SN2 reaction. Good leaving groups are weak bases that can stabilize the negative charge that develops on them after leaving. Common good leaving groups include halides (Cl –, Br –, I –), tosylate (TsO –), and triflate (CF 3SO 3–).
Questions Often Asked
What is the key characteristic of a strong nucleophile in SN2 reactions?
Strong nucleophiles possess a high electron density and a negative charge or a lone pair of electrons that can readily attack the electrophile.
How does solvent polarity influence the rate of SN2 reactions?
Polar solvents stabilize charged intermediates, leading to faster SN2 reactions. Conversely, nonpolar solvents hinder the formation of charged intermediates, resulting in slower reactions.
What is the effect of steric hindrance on SN2 reactions?
Bulky substituents around the electrophilic carbon center create steric hindrance, which impedes the approach of the nucleophile and slows down the reaction rate.